When I read the title of this paper – Arsenical-maleimide for the Generation of New Targeted Biochemical Reagents – I thought you’ve got to be kidding, but having perused the abstract, I realised that I had much to learn.
Inorganic arsenicals (my favourite new word) including arsenic trioxide have been used for millennia to treat cancers and a range of other conditions. In the beginning of the 20th century work Ehrlich and Hata carried out work on designing organo-arsenicals for the treatment of syphilitics and introduced Arsphenamine, as the first rational chemotherapeutic (Fig 1). Their effort was the first organised team effort to optimise the biological activity of a lead compound through systematic chemical modification. Arsphenamine is unstable in air and was administered by injection once dissolved in several hundred ml of water. The side effects attributed to Arsphenamine was proposed to stem from improper administration, leading to the rigorous Ehrlich to conclude ‘the step from the laboratory to the patient's bedside ... is extraordinarily arduous and fraught with danger.’ But why am I telling you this, WATCH THE FILM!
Inorganic arsenicals (my favourite new word) including arsenic trioxide have been used for millennia to treat cancers and a range of other conditions. In the beginning of the 20th century work Ehrlich and Hata carried out work on designing organo-arsenicals for the treatment of syphilitics and introduced Arsphenamine, as the first rational chemotherapeutic (Fig 1). Their effort was the first organised team effort to optimise the biological activity of a lead compound through systematic chemical modification. Arsphenamine is unstable in air and was administered by injection once dissolved in several hundred ml of water. The side effects attributed to Arsphenamine was proposed to stem from improper administration, leading to the rigorous Ehrlich to conclude ‘the step from the laboratory to the patient's bedside ... is extraordinarily arduous and fraught with danger.’ But why am I telling you this, WATCH THE FILM!
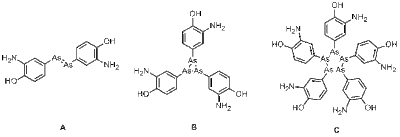
Figure 1: The structure of Arsphenamine has been proposed to be akin to an azobenzene (A), but mass spectral studies published in 2005 suggest it is actually a mixture of the trimer B and the pentamer C.
The use of arsenicals declined with the advent of antibiotics and other drugs, but perhaps due to desperation arsenicals are back on the map. As2O3 has been shown as an effective treatment of promyelocytic leukaemia and several organoarsenicals have made it to clinical trials for the treatment of leukaemia and solid tumours. The consensus is that biological activity of As(III) species stems from their coordination to thiols. Therefore the challenge for arsenicals is for efficient targeting to maximise specificity. Thorpe et al take up this challenge by designing maleimide arsenicals, that can be efficiently conjugated to thiol bearing proteins and thus delivered to specific targets (Fig 2).
Figure 2: Synthesis of maleimide D and its conjugation to a thiol bearing protein.
They demonstrate the selectivity of the maleimide for thiols via a conjugate addition over the coordination of thiols to the As(III) atom by titration of the X with GSH, a decline in the absorbance at 320 nm (as observed with phenyl maleimide) with an end point of D/GSH of 1:1. The reaction was rapid and was also demonstrated to work on a model protein containing one solvent exposed cysteine residue (confirmed by MS).
To test the selectivity of a conjugate the arsenical was reacted with a thioredoxin (trx). Thioredoxins are a set of redox enzymes that have a CXXC motif that is able to perform redox reactions with other proteins. Thioredoxin is kept in its reduced form by thioredoxin reductase which uses NADPH as its hydride source. The conjugate was able to inhibit the activity of the reductase to a greater degree than other inorganic and organo arsenicals.
Conjugate probes were also tested as inhibitors of protein disulphide isomerase (PDI), which has been suggested as a target for antiviral and anticancer therapies. They also contain active CXXC domains which are responsible for redox transformations, for example, some intracellular PDIs act as chaperones which assist in the correct folding of protein targets via disulphide bond formation whilst some membrane bound PDIs modulate extracellular redox poise. An arsenical conjugate was prepared by addition of D to the PDI substrate pancreatic ribonuclease A (RNase). The RNase has 4 disulphide bonds so mutants were prepared to conjugate the Arsenical at various positions. The conjugates were then able to inhibit the PDI. Inhibition in the assay was also observed in the presence of 5 uM GSH as the authors recognised that extracellular GSH concentrations can reach 10 uM.
Unfortunately, I have skipped over a lot of the experimental assay details but I think Thorpe et al have concisely demonstrated the potential of selectively inhibiting catalytic thiols using arsenical conjugates. Arsenicals have the potential to be delivered selectively using cell penetrating peptides and thiol containing dendrimers. The maleimide arsenicals developed here will be subject to reverse Michael reactions over time, but this could be used for the controlled release of cytotoxic species. The future looks very interesting for arsenicals and I will keep an eye out for arsenical publications with less fear and trepidation.

Here's another interesting fact: the arsphenamine you mentioned is also known as "606" because it was the 606-th compound tested in the search for syphillis treatment.
ReplyDelete